Electrochemical CO2 Conversion
Reducing greenhouse gas emissions is widely acknowledged as a major international research priority. To avoid the effects of climate change, the scientific community has set a target of a <2°C increase in the average global temperature relative to the pre-industrial times. Achieving this target will require reducing total carbon dioxide (CO2) emissions by 80% by 2050, and up to 1,000 Gt of CO2 needs to be cumulatively captured and stored by 2100.
Renewable energies, such as wind and solar, are widely distributed and, taken together, could exceed today’s global electricity demand > 10,000-fold. However, the intrinsic intermittency of solar or wind resources limits their further deployment as replacements of fossil fuels.
Electrochemical CO2 conversion (ECC) to fuels and chemicals addresses the CO2 emission and renewable energy storage problems simultaneously. Existing infrastructure can be leveraged to take advantage of these chemicals and fuels, and recycling CO2 results in a low- or zero-carbon fuel cycle.
Developing Electrocatalysts
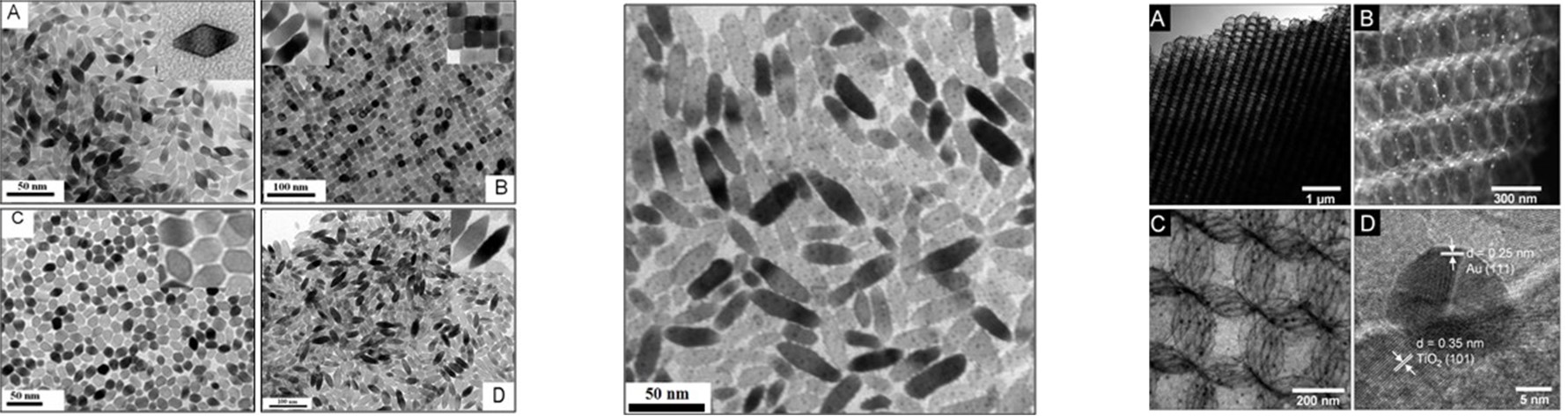
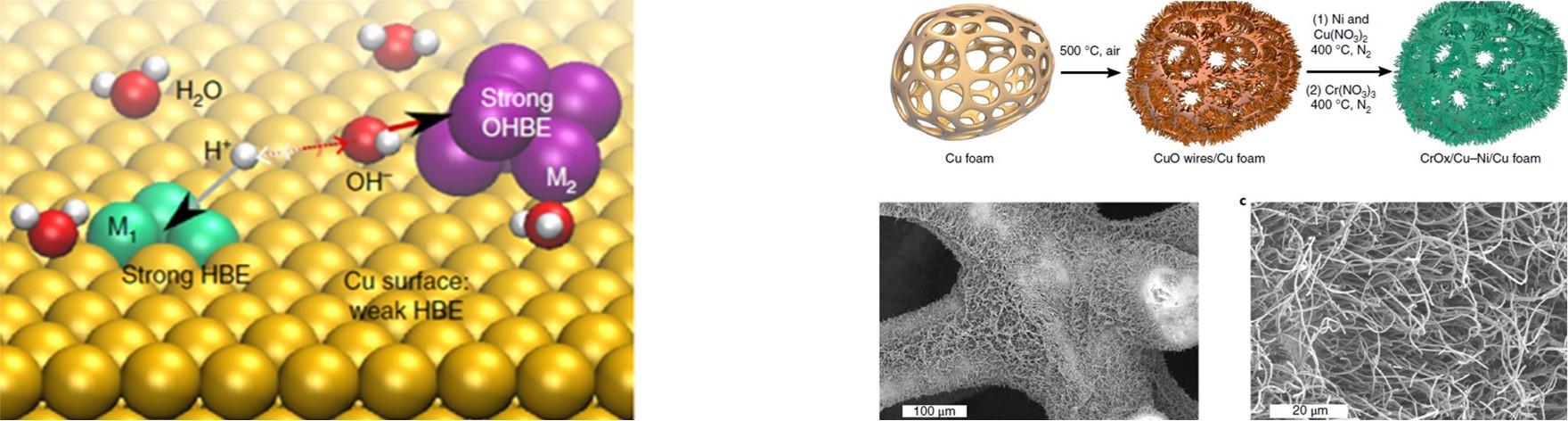
Electrocatalysts play a critical role in an electrochemical process because they lower the activation energy needed to increase the rate and efficiency, and they govern the selectivity of the chemical transformation involved. We are focusing on developing multicomponent nanostructured catalysts for producing multiple carbon products, such as ethylene and ethanol from CO2. Nanostructured catalysts often show enhanced activity compared to the bulk materials owing to their unique morphological, electronic, and chemical surface properties. We control the morphology and crystallinity of the catalysts to expose the desirable active sites, such as surface defects and grain boundaries. We also explore and tune multicomponent catalysts, including metal alloys and metal/oxide hybrids to optimize their electronic properties.
Example:
C. T. Dinh*, A. Jain*, F. P. G. de Arquer*, P. De Luna, N. Wang, X. Zheng, J. Cai, O. Voznyy, B. Zhang, M. Liu, D. Sinton, C. Ethan, E. H. Sargent,
Multi-Site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules,
Nature Energy, 2019, DOI: 10.1038/s41560-018-0296-8
Developing Gas Diffusion Electrodes
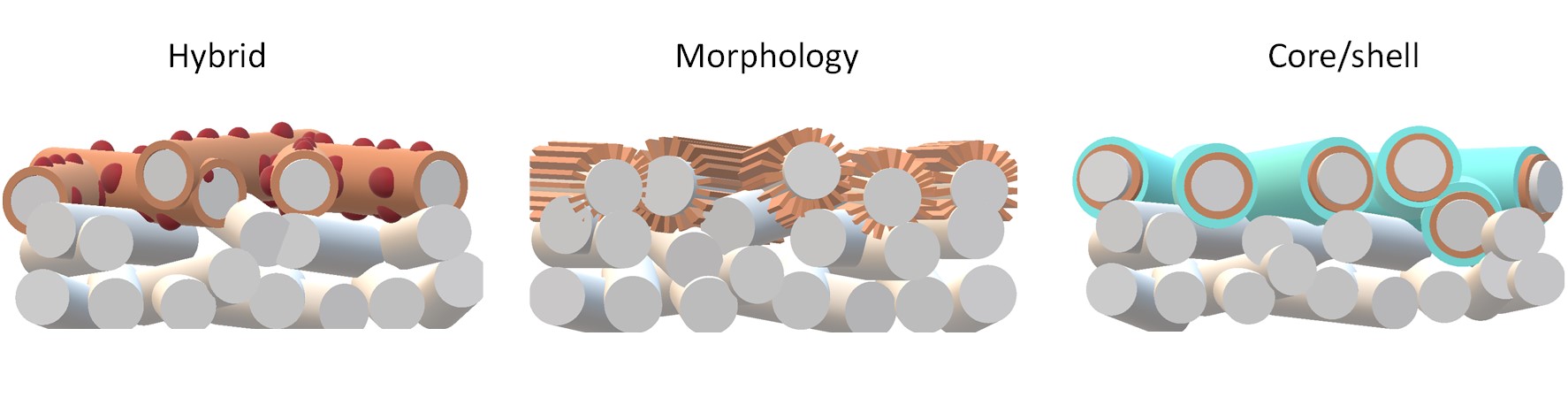
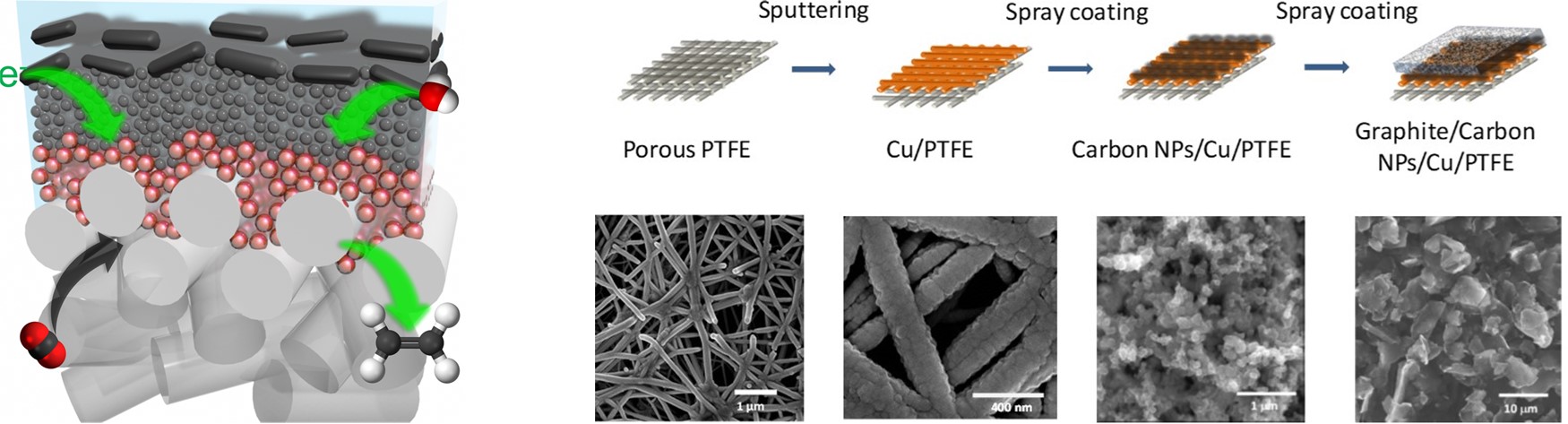
In a gas-phase electrochemical CO2 conversion (ECC) system, a CO2 reduction catalyst is deposited onto a porous gas diffusion layer (GDL), forming a gas diffusion electrode (GDE). Unlike in a liquid-phase ECC system, here CO2 reaches the catalyst through the GDL in gas phase, thus reducing the required diffusion length and enabling high operating current densities. The present cathodes of the gas-phase ECC, however, become unstable at high current densities leading to a failure of ECC within a few hours of operation. We develop efficient GDE for CO2 conversion by tuning the porosity, morphology, hydrophobicity, and electrical conductivity of GDL, its essential features, in combination with electrocatalyst design, to achieve a viable ECC technology.
Example:
C. T. Dinh*, T. Burdyny*, M. G. Kibria*, A. Seifitokaldani*, C. M. Gabardo, F. P. G. de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton, E. H. Sargent,
CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface,
Science, 2018, 360, 783-787.